Hot-Dip Galvanizing to ASTM A123 Fact Sheet
Hot-dip galvanizing (HDG) is the process of dipping fabricated steel into a kettle or vat containing molten zinc. While the steel is in the kettle, the iron in the steel metallurgically reacts with the molten zinc to form a tightly-bonded alloy coating that provides superior corrosion protection to steel.
The hot-dip galvanizing (HDG) process consists of three basic steps:
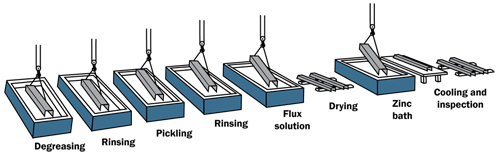
- Surface Preparation
- Galvanizing
- Inspection
Surface Preparation
Surface preparation is a critical step in the application of any coating. The galvanizing process has its own built-in means of quality control because zinc will not react with an unclean steel surface. Any failures or inadequacies in surface preparation will be immediately apparent when the steel is withdrawn from the zinc bath because the unclean areas will remain uncoated, and immediate corrective action can be taken. Surface preparation for galvanizing consists of three steps:
Degreasing/ Caustic Cleaning
A hot alkali solution, mild acidic bath, or biological cleaning bath removes organic contaminants such as dirt, paint markings, grease, and oil from the metal surface. Epoxy, vinyl, asphalt, or welding slag, which cannot be removed by degreasing, must be removed before galvanizing by grit-blasting, sand-blasting, or other mechanical means.
Pickling
A dilute solution of heated sulfuric acid or ambient hydrochloric acid removes mill scale and iron oxides (rust) from the steel surface. As an alternative to or in conjunction with pickling, this step can also be accomplished using abrasive cleaning or air blasting sand, metallic shot, or grit onto the steel. As long as the surface of the steel is completely cleaned to bare metal and all non-metallic substances removed, the galvanizing reaction is indifferent to the manner of cleaning.
Fluxing
The final surface preparation step in the galvanizing process, a zinc ammonium chloride solution, serves two purposes. It removes any remaining oxides from the surface of the steel, and deposits a protective layer on the steel to prevent any further oxides from forming on the surface prior to immersion in the molten zinc.
Galvanizing
During the actual galvanizing step of the process, the material is completely immersed in a bath of molten zinc. The bath chemistry is specified by ASTM A123 and requires at least 98 % pure zinc by weight maintained at a temperature above the melting point of zinc. The zinc metal in the bath must meet the requirements of ASTM B6 or ASTM B960.
While the steel is immersed in the kettle, zinc reacts with iron in the steel to form a series of metallurgically-bonded zinc-iron inter-metallic alloy layers, commonly topped by a layer of pure zinc metal from the bath. Once the fabricated items' coating growth is complete, it is withdrawn slowly from the galvanizing bath, and the excess zinc is removed by draining, vibrating, and/ or centrifuging. The metallurgical reaction will continue after the materials are withdrawn from the bath, as long as the steel part remains near bath temperature. Galvanized articles are cooled either by immersion in a passivation solution or water, or by being left in open air.
Inspection
The inspection of hot-dip galvanized steel is simple and quick. The two properties of the hot dip galvanized coating most closely scrutinized are coating thickness and appearance/ surface condition. A variety of simple physical tests can be performed to determine thickness, uniformity, adherence, and appearance.
Fabricator -- Controllable Variables
To promote the best possible outcome from the hot-dip galvanizing process, the fabricator should consider controlling several variables which impact the coating appearance and characteristics.
Steel with properly designed vent and drain holes, cropped gusset plates, and seal-welded overlapping surfaces as prescribed by ASTM A385 will most often result in a galvanized coating without excess zinc buildup inside or outside the steel article. Optimal immersion and removal speed from the molten zinc bath is a slow, steady rate. This allows even heating during immersion and even freezing of the zinc during removal. Too few or too small drain and vent holes make immersion and removal very slow and inefficient, and the result is a very uneven coating. Poor seal welds or stitch welding causes 'blowout' of trapped chemicals from the overlapped surfaces and steel around the weld area may remain un-galvanized.
Welded steel free of flux and slag and the use of weld rod similar in chemistry to the steel being welded. Residual flux and slag are not always visible to the galvanizer and are not removed during the chemical cleaning steps. The result is un-galvanized areas around the weld. If the weld rod is dissimilar in chemistry to the steel being welded, the zinc reaction with the weld metal will likely yield a different appearance and different coating thickness compared to the steel members.
Stress-relieving steel/ weld bead near the area of flame-cut cope edges. The thermal gradient inherent in the galvanizing process affects the residual stresses in structural steel caused by flame cut copes. If there is no stress-relieving prior to galvanizing, the cope cut areas may exhibit cracks after galvanizing. The fabricator may alternatively place weld bead on the beam web adjacent to the cope area, thereby relieving some of the residual stress in this area.
Minimize cold-working, but if necessary, do it in accordance with recommended bend radius. Cold-working induces stress into structural steel and the excess residual stress can age the steel at galvanizing temperatures, thereby changing the steel properties. The heat of the galvanizing process accelerates the appearance of cracks but does not cause the cracks.
Use removable steel tags, weld bead, or stenciling to mark fabricated pieces. Paint, lacquer, and some marking pen material are not removed in the cleaning process and must be removed mechanically prior to beginning the galvanizing process.
Fabricator -- Uncontrollable Variables
Steel production is not a precise process in terms of resulting chemistry and an even distribution of elements throughout. Some fabrications combine steels of varying chemistry and thickness. Both influence the outcome of the galvanized coating appearance and the implications for the fabricator are as follows.
Fabrications using steel with two or more different chemistries The metallurgical reaction rate between the molten zinc and each of the steel types may be quite different and result in both a different coating thickness and a different appearance, despite each part of the assembly being immersed in the same bath for the same amount of time. There is no way to modify the immersion time so as to produce a uniform coating thickness or appearance.
Fabrication volume insufficient to be galvanized in a compatible batch To be as productive and efficient as possible, galvanizers will combine several jobs, which may contain quite different steel thickness and chemistries amongst its articles, and use wire to suspend the steel from a rack used to immerse all of the parts into the cleaning and molten zinc baths at one time. There is no way to predict or ensure the appearance of the various steel pieces will be consistent. Even if all the steel articles were of the same thickness and an exact immersion time for that specific thickness was used, the coating thickness and appearance from piece to piece may be quite different.
Steel with a homogeneous chemistry where all elements are evenly distributed throughout the steel article Both the continuous cast and traditional iron ore/coke/limestone methods of producing steel do not guarantee the chemistry of the steel as reported on the mill test report is consistent throughout a beam, plate, or tube. In fact, it is normal for the chemistry from Point A to Point Bon a structural steel piece to be slightly different. This difference in silicon and/or phosphorous, the two elements in steel causing the most reactivity between the iron and the molten zinc, often results in a different galvanized coating thickness and/or appearance on the same piece of steel. Even if the chemistry were precisely known and 100% homogenous throughout the piece of steel, having a prescribed immersion time and molten zinc temperature does not guarantee a predictable outcome. There are other unknown elements in the steel and possible rolled or fabricated stresses induced in the steel which may cause an area of matte finish adjacent to an area with a bright and shiny finish.
Galvanizer Variables
Extensive research and testing by the galvanizing industry has resulted in best practices which are not 100% predictive of coating appearance and thickness, but deliver the most consistent quality within the parameters the process allows for the variety of materials being galvanized. This is what we know.
The addition of a small amount of nickel to the molten zinc bath helps to control the reactivity between iron and silicon as well as iron and phosphorous for some steel chemistries but a specific range of coating thickness is not predictable for any thickness of steel.
The addition of aluminum to the molten zinc bath generally results in a shinier zinc coating, but does not guarantee there will be no matte coating appearance on a part or all of a steel piece.
There is no way to measure the degree of mill scale and corrosion on a piece of steel prior to immersion in the cleaning process tanks. Thus, the amount of time for the steel to reside in the acid bath is determined by experienced operators and is not available from a table of values.
Pickling with hydrochloric or sulfuric acid are both effective means of removing mill scale and rust. Over-pickling is possible when using sulfuric acid but the resulting zinc coating is only marginally thicker than had the pickling time been exact. Galvanizers are careful to not over-pickle because applying excess zinc makes the production less economical.
Immediate water or passivation quenching after the steel is removed from the galvanizing bath stops the reaction between the iron and zinc, usually resulting in a shinier coating, but not always. For tubular products, the quenching cools the material quickly and typically prevents peeling, i.e. the separation of the pure zinc outer layer of the coating from the alloy layers. Based on symmetry and steel thickness, some fabrications cannot be water quenched due to concerns related to distortion.
Galvanizers regularly test and adjust the strength of their acid and flux solutions, and either completely change them out or employ a filtering system to maintain their effectiveness. Any variance from the optimal strength and/or cleanliness only affects the efficiency of the process and not the quality of the galvanized coating. If the flux fails, then this is immediately visible as un-galvanized areas on the fabrication right after removal from the zinc bath. Galvanizers regularly remove zinc-iron compounds called dross from the bottom of the galvanizing kettle where they settle over time. Effective "drossing" is important to maintain the kettle in proper working order and also minimizes the amount of dross that could potentially end up on the galvanized steel as it is removed from the kettle. The frequency of "drossing" the kettle is a function of how much steel volume is being processed and the cleanliness of the flux cleaning solution. This is an involved process and production cannot be done while "drossing;" thus, galvanizers are flexible on scheduling the drossing to ensure they meet customer demand.
Prior to steel articles and fabrications being removed from total immersion in the zinc bath, galvanizers paddle zinc skimmings (zinc-oxide) to the side of the kettle so very little, if any, skimmings settle on top of the newly galvanized surface during removal from the bath. Because the oxidation of the molten zinc is a continuous process, it is not always possible to have all surfaces completely free of skimmings.
